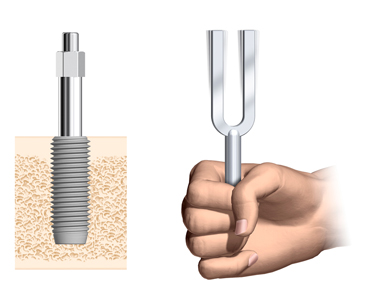
Resonance frequency & ISQ
The resonance frequency depends on the dimensions and material of the Smartpeg, and the “boundary conditions” between the SmartPeg and the Implant. Since the connection between the SmartPeg and the implant is good, “boundary conditions” means how fix the implant is – the implant stability. Thus, if the dimensions and material of the SmartPeg is set, the resonance frequency of the SmartPeg will depend on only the implant stability.

Height over bone level
If the stability of the implant is measured above the bone level, it will be lower than if measured at bone level. This is because the part of the implant above bone will act as a prolonged part of the SmartPeg, and the resonance frequency (and ISQ) will therefore be lower. If there is marginal bone loss around the implant, it will show as a lower ISQ. If the implant is prolonged with an abutment the same effect will be apparent.
Different SmartPeg types
Due to the reasons above, it is necessary that the connection between the SmartPeg and implant is good; the surfaces have to meet each other around the implant, and they should not be too large. They also have to have the right type of thread. Since implants with many different geometries are available, it is necessary to use SmartPegs that meet these criteria, and therefore a variety of SmartPeg types exist. SmartPeg types differ in length, thread type, diameter and connection surfaces – some are flat and some have a conical attachment. Many implants have similar prosthetic attachments, and therefore it is sometimes possible to use the same SmartPeg type for different implant types.
It is advantageous that two different implants that use different SmartPegs give similar ISQ when they have the same stability. However, it is impossible to get an exact match, and the reason is that there is nothing to compare the ISQ to.
Even if the method below -with curing plaster- is used, any difference in ISQ can be due to the fact that two SmartPeg types are used, that the same SmartPeg type is used but attaches to the implants somewhat differently, or due to that the implants really have different stability. Therefore, we do not recommend or encourage comparing absolute values between implant systems, or drawing clinical conclusions from one implant system, and transferring or using those conclusions with another implant system. Relative ISQ values is a different issue. Since the difference between SmartPeg types is made as small as possible (see below about designing SmartPegs), it is possible to compare relative values, i.e. the change in ISQ from one point in time to another.
Designing new SmartPegs
When a Smartpeg for a new implant is designed, it is adapted to fit the geometry of the implant, have the right thread and to be clinically user friendly. In order to check whether the design will give similar or comparable ISQ to another implant, the new design and a reference is tested in curing plaster. This method is also used if an existing SmartPeg, intended for another system, is used for a new implant. The reference is selected to be an implant that has a geometry that is similar to the tested implant.
If the ISQ develops in a similar way between the tested SmartPeg and the reference, the new design is approved.
By using this method, it ensures that two different implants will not deviate too much in ISQ, given the same stability. However, any difference will be due to the mechanical design of the implant and/or the differences in SmartPeg dimensions and/or the way the SmartPeg attaches to the implant. An estimation is that ISQ can differ approximately 5 ISQ units in the clinically relevant region (between 45 and 75 ISQ) due to these reasons.
Using Osstell and ISQ values to compare implant performance
Caution has to be used when comparing the ISQ of different implants, especially in in-vitro models with artificial bone. The ISQ is a measurement of the stiffness of the bone-implant interface and the bone stiffness close to the implant, but is also influenced by the distance from bone level to the top of the implant. The ISQ-results from in-vitro tests do not necessarily reflect the in-vivo test result, and especially if the differences are small (<5 ISQ units).
Therefore, Osstell does not recommend or support comparing absolute values between implant types. To make comparisons, Osstell recommends using an in-vivo model and also comparing the relative development of ISQ over time, instead of absolute values.